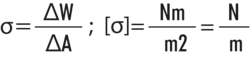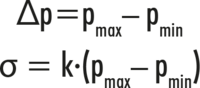The Surface tension σ (sigma) is a property of a liquid (e.g. water) at the interface with a gas (e.g. air). It is important in many industrial processes, e.g. in the Wetting of surfaces.
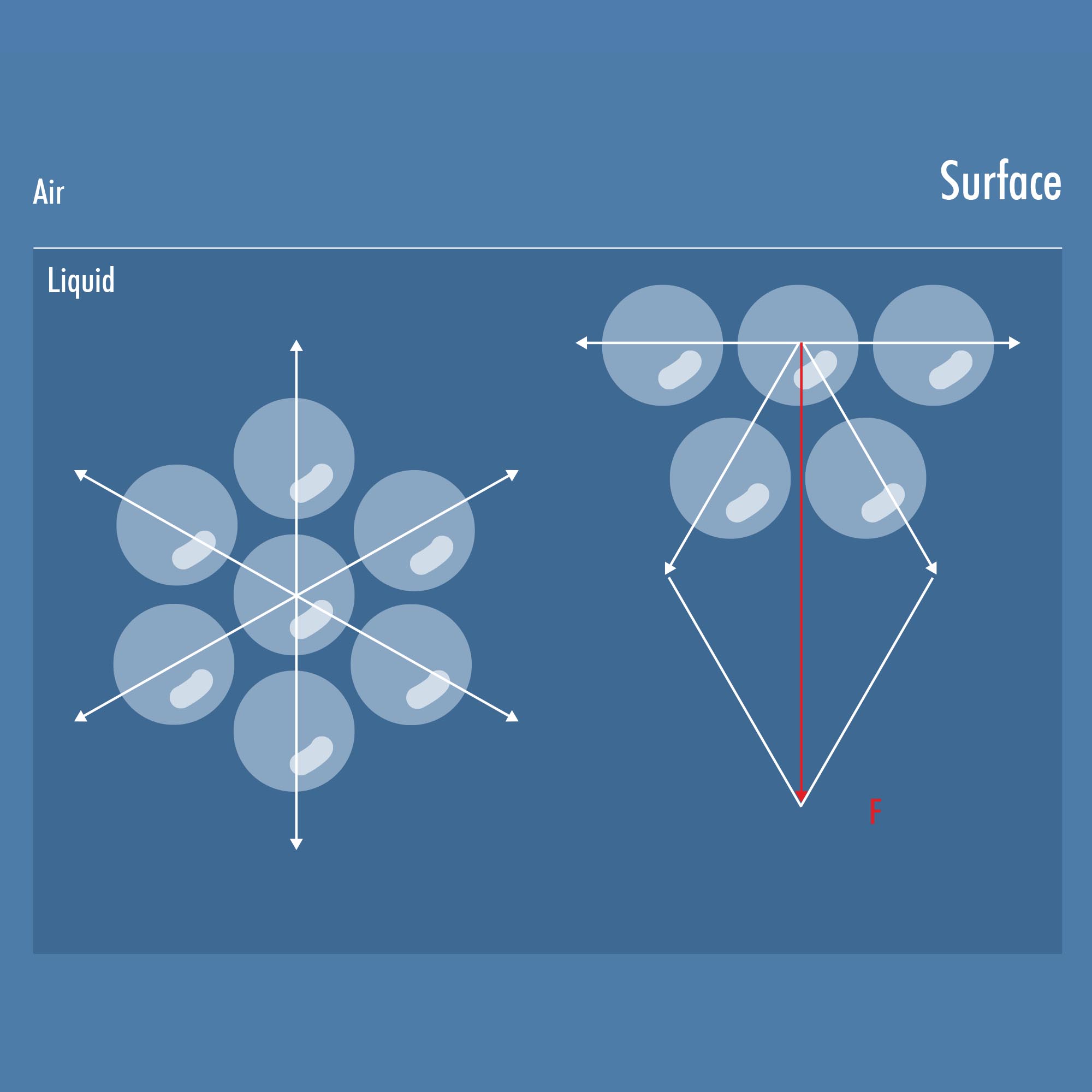
In a liquid, the molecules interact with each other. Cohesive forces cause the molecules to attract each other. These cohesive forces create tension on the surface of the liquid, similar to a skin that always strives to keep the surface area as small as possible. This effect is particularly noticeable in water droplets, whose round shape is created by these inwardly directed forces.[1].
Surface tension is described as the work that must be done to increase the surface area by a certain amount:
Surface tension is usually expressed in mN/m or dyne/cm. The surface tension of water at 20 °C is 72.75 mN/m (cf. ethanol 22.55 mN/m). For many aqueous applications, the surface tension must be reduced. Surfactants are used here.
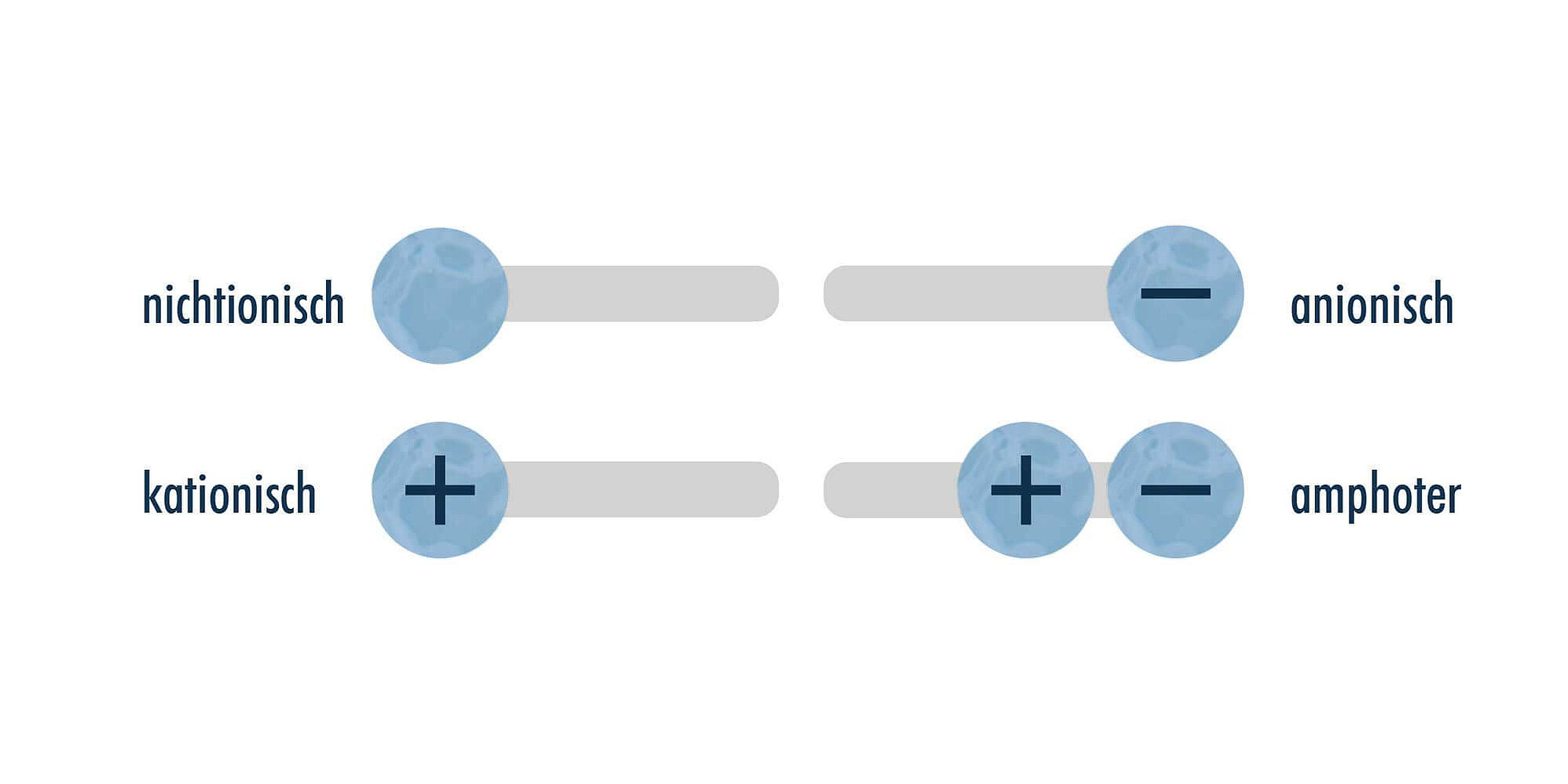
Surfactants are a group of chemical compounds consisting of two opposing molecular parts: a nonpolar, hydrophobic hydrocarbon part (shown in yellow in the image) and a polar, hydrophilic head group (shown in blue in the image). Depending on the type of head group, there are non-ionic, anionic, cationic and amphoteric surfactants. Non-ionic and anionic surfactants are mainly used in aqueous parts cleaning applications. Cationic surfactants are typically used in fabric softeners or as surface water repellents. Amphoteric surfactants are found in many skin and hair cleansing products.
The two molecular parts behave very differently towards water: the hydrophobic part repels water, while the hydrophilic part is attracted to water. Due to these contrasting components, they migrate to surfaces in an aqueous environment[2].
Such surfaces include interfaces like water-air, water-solid, or water-oil. At the surface, surfactants occupy spaces between water molecules, disrupting cohesion and thus reducing surface tension. This behaviour is known as surface activity. The movement of surfactants towards a surface is time-dependent and is influenced by the type and concentration of the surfactant, temperature, and the surrounding liquid (matrix effects).
The Surface-active behaviour of surfactants, also known as wetting agents, is deliberately exploited in various technical applications to achieve multiple effects, such as:
- Wetting surfaces
- Removing oils and fats from surfaces
- Emulsifying oil in water and dispersing pigments, etc.
- Foam formation or prevention
- Droplet formation and adjusting droplet size
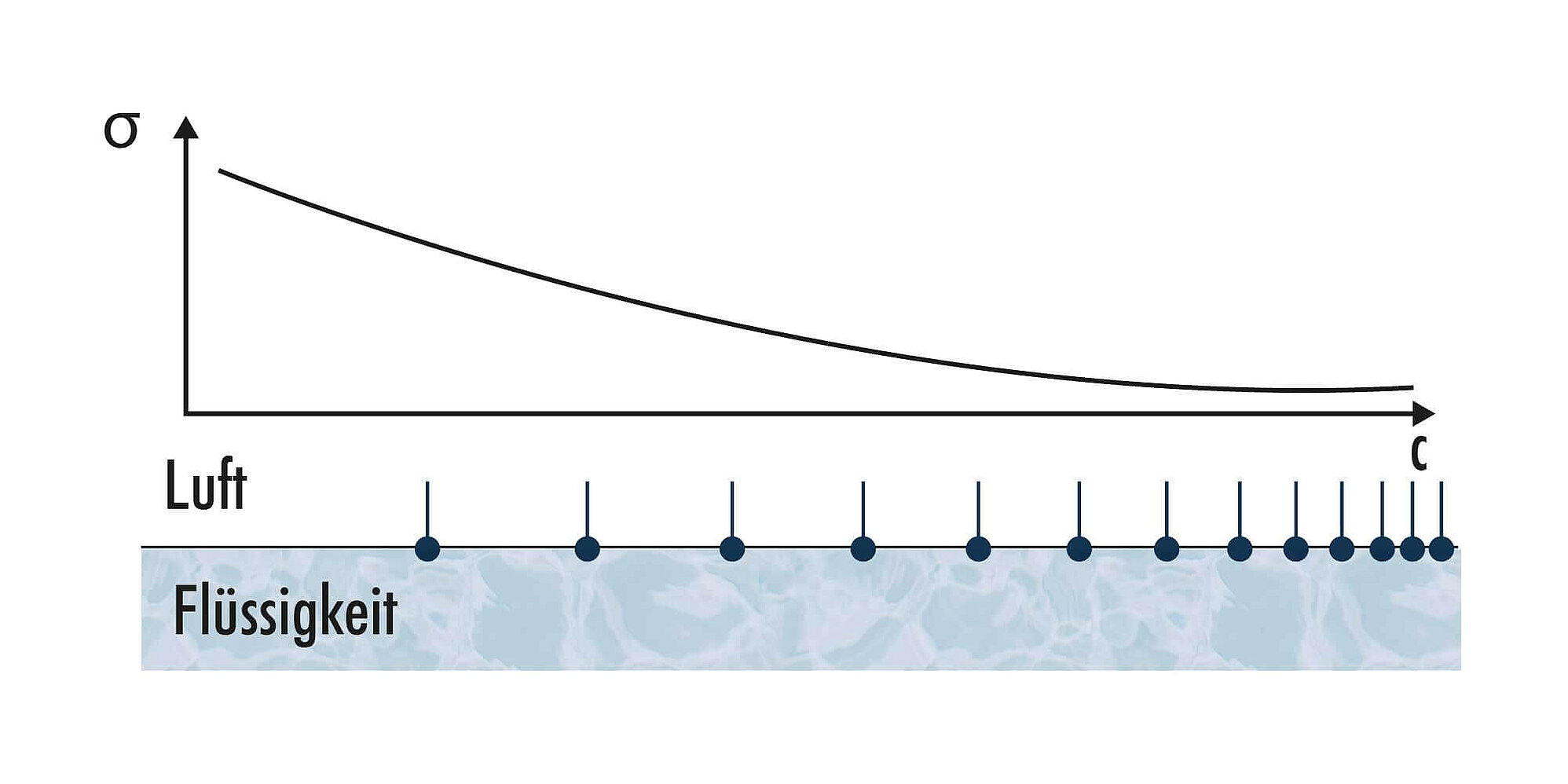
As surfactants reduce the surface tension, the measured surface tension can be used to determine the content of free surfactants available for technical applications.
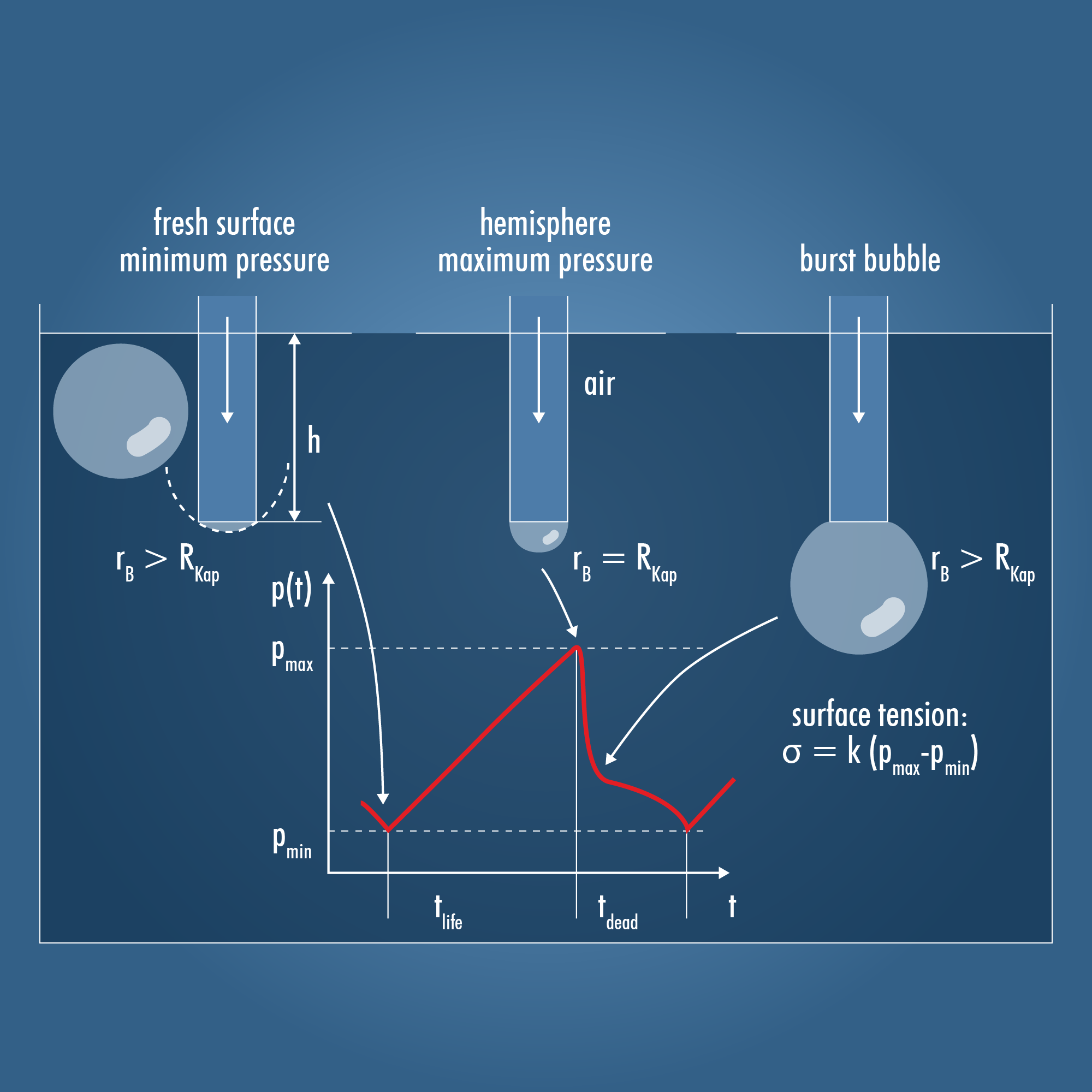
Methods for measuring surface tension must intentionally increase the surface area, typically through pulling or pushing. In the bubble pressure method, this is achieved by creating an air bubble at the tip of a Capillary. All SITA tensiometers operate using this method, ensuring that the measurements are comparable.
Air is introduced into the liquid through a capillary, forming an air bubble at the capillary tip. The pressure in the (air) volume stream is continuously measured. The air bubble goes through three stages (as shown in the image above):
- Shortly after a bubble detaches from the capillary, the interfacial tension draws the liquid surface into the capillary due to the capillary effect, creating a pressure minimum (top left).
- As more air is introduced, the bubble inflates until the bubble radius equals the capillary radius, reaching a pressure maximum (top centre). This is known as a hemispherical bubble.
- With further air introduction, the bubble rapidly expands (top right). The volume increases, and the pressure decreases. Shortly after, the bubble detaches from the capillary, and the cycle repeats.
The difference between the minimum pressure pmin and the maximum pressure pmax is proportional to the surface tension σ according to the Young-Laplace equation:
The calibration factor k includes the fluid dynamic properties of the capillary and system parameters of the Tensiometer, determined during calibration. This systemic factor k is evaluated based on various bubble lifetimes tlife during multi-point calibration.
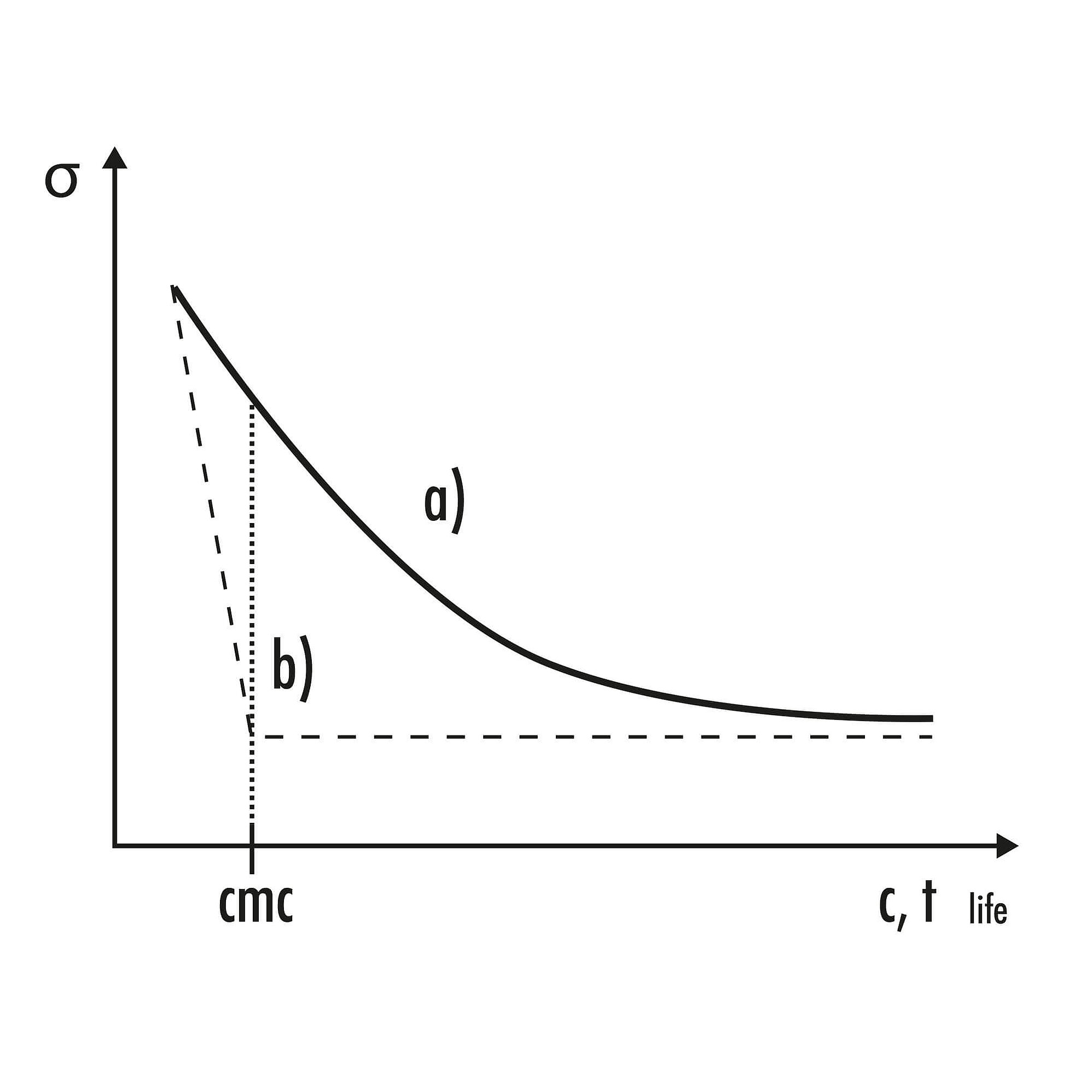
SITA tensiometers use the bubble pressure method to measure surface tension. In this method, the measured dynamic surface tension always depends on the parameter Bubble lifetime tlife, which corresponds to the wetting time or the surface age. The bubble pressure method is classified as a dynamic measurement method.
Other dynamic measurement methods include the drop volume method using a stalagmometer or interfacial rheology with a hanging drop. Methods for measuring static surface tension include the Du Noüy ring method and the Wilhelmy plate method.
In dynamic methods such as bubble pressure tensiometry, a quasi-static value of surface tension is reached in comparison to static methods only at high surfactant concentrations c or after long bubble lifetimes tlife. Therefore, the necessary clear correlation between surface tension and concentration required for concentration determination is present even above the critical micelle concentration cmc[2]. For the surface tension measurement of surfactant-free, non-surface-active liquids (e.g., solvents), there is no difference between dynamic and static methods.
If surface tension is mentioned in measurement specifications or literature without specifying a surface age or the parameter bubble lifetime tlife, it should be assumed to be a static surface tension.
1 In water, hydrogen bonds predominantly act, while in nonpolar liquids, Van der Waals forces are responsible for cohesion. These intermolecular forces hold the molecules together. However, the properties of the liquid's surface layer differ from those of the bulk phase. The attractive force from the bulk phase still acts on the molecules of the liquid near the interface, resulting in a net force directed towards the interior of the liquid.
2 More precisely, the carbon chain of the hydrophobic part disrupts the formation of hydrogen bonds, causing this part of the surfactant to be pushed away from the water. Since everything in chemistry strives for a state of lower energy, this pushing continues until the carbon chain is completely oriented away from the water. This occurs when it either protrudes out of the interface or towards a similarly hydrophobic oil droplet. If there is no interface nearby, Micelles form. These are small surfactant droplets with hydrophobic ends pointing inward and hydrophilic head groups pointing outward. The specific concentration at which this process starts is called the critical micelle concentration (cmc).